
Research

1. Lithium-ion Batteries
The fight against climate change requires a global shift to electrified mobility and renewable electricity generation. Lithium-ion batteries are the key technology to enable this transition. Today's lithium-ion batteries can use various materials in the positive and negative electrode, all of which have their advantages and disadvantages. In the following paragraphs you will find out how we make lithium-ion batteries even better.

The search for better battery materials
While the battery industry is rapidly scaling up cell production in new Gigafactories, intensive research and development is needed to lower the cost of modern battery cells, increase their lifetime and energy density, and improve their safety. In particular, a shift to more sustainable, cost-effective and abundant elements like iron and manganese, and eco-friendly manufacturing technologies are needed to allow for continued growth in an environmentally friendly way. The electrification of the transport sector requires sustainable lithium-ion batteries with an energy-density high enough to provide sufficient driving range, and a cost low enough to afford mass adoption of battery electric vehicles.
In our lab, we make iron and manganese-based materials for the positive electrode by melt synthesis (see video, reference [1] and Figure 1), study graphite and silicon for the negative electrode, and develop sophisticated electrolyte formulations tailored for battery lifetime, performance and safety.

Figure 1. A lithium-ion battery cathode material synthesized in our lab before processing into an electrode.
The importance of battery lifetime
A large-scale shift towards sustainable and cost-effective lithium-ion batteries requires intensive research into battery lifetime. If lifetimes are short and cell production rates cannot increase quickly enough, future battery production will predominantly support the replacement of the ever-dying batteries in cellphones, laptops, and electric vehicles rather than supporting the increase of global storage deployment and enabling the usage of intermittent renewable energy sources. For example, global battery production capacity in 2022 is assessed at ~1 TWh, projected to be 2.5 TWh in 2025 and as high as 6 TWh by 2031. If the lifetime of those batteries is 3 years, then the batteries constructed in 2022 will reach end-of-life in 2025. Of the 2.5 TWh produced in 2025, 1 TWh must be utilized to replace the batteries made in 2022 that will have failed, and only the remaining 1.5 TWh can be used to increase the global energy storage deployment. Hence, decades long lifetime of sustainable lithium-ion batteries is crucial for achieving the required total energy storage deployment of 400 TWh by ~2050, a timeframe set out in the Paris Agreement.
To address this challenge, our research team at Dalhousie works on sustainable, long-lifetime lithium-ion batteries [2]. We quantify the rate of side reactions in our advanced battery chemistries with high-end characterization tools, e.g., ultra-high precision coulometry (UHPC) and on-line electrochemical mass spectrometry (OEMS). This helps us to understand and ultimately stop parasitic reaction that limit the lifetime, performance, and safety of battery cells. Battery aging can be accelerated by high temperature testing and dramatic changes to the electrolyte are observed (see Figure 2).

Figure 2. Lithium-ion battery electrolytes extracted from cells after cycling at different temperatures. Specialized molecules we add to the electrolyte (additives) can prevent degradation reactions that lead to color change.
Example of a parasitic reaction in batteries
Imagine that you charge your laptop to 100% and a few days later the battery only shows 90% even though you did not use it. Our group has found examples of parasitic reactions in commercial batteries that can lead to such unwanted self-discharge. At high temperature and in the absence of effective electrolyte additives (small specialized molecules that passivate the electrode surfaces), a so-called redox shuttle can be created inside the battery, which leads to rapid self-discharge [3].
A redox shuttle is a chemical compound that first gains electrons on the negative electrode, migrates to the positive electrode to give up these electrons, and then returns to the negative electrode to repeat this process (see Figure 3). Simultaneously, lithium ions move from the negative electrode to the positive electrode to ensure charge neutrality. Since a battery is fully charged when all lithium is in the negative electrode, the redox shuttle effectively discharges the battery. The ability of lithium-ion cells to hold their charge at elevated temperature is especially important for stationary storage of renewable energy, as such systems are often deployed in hot climates favorable for solar energy. Our discovery can help to eliminate such parasitic reactions by modifying the battery cell chemistry.
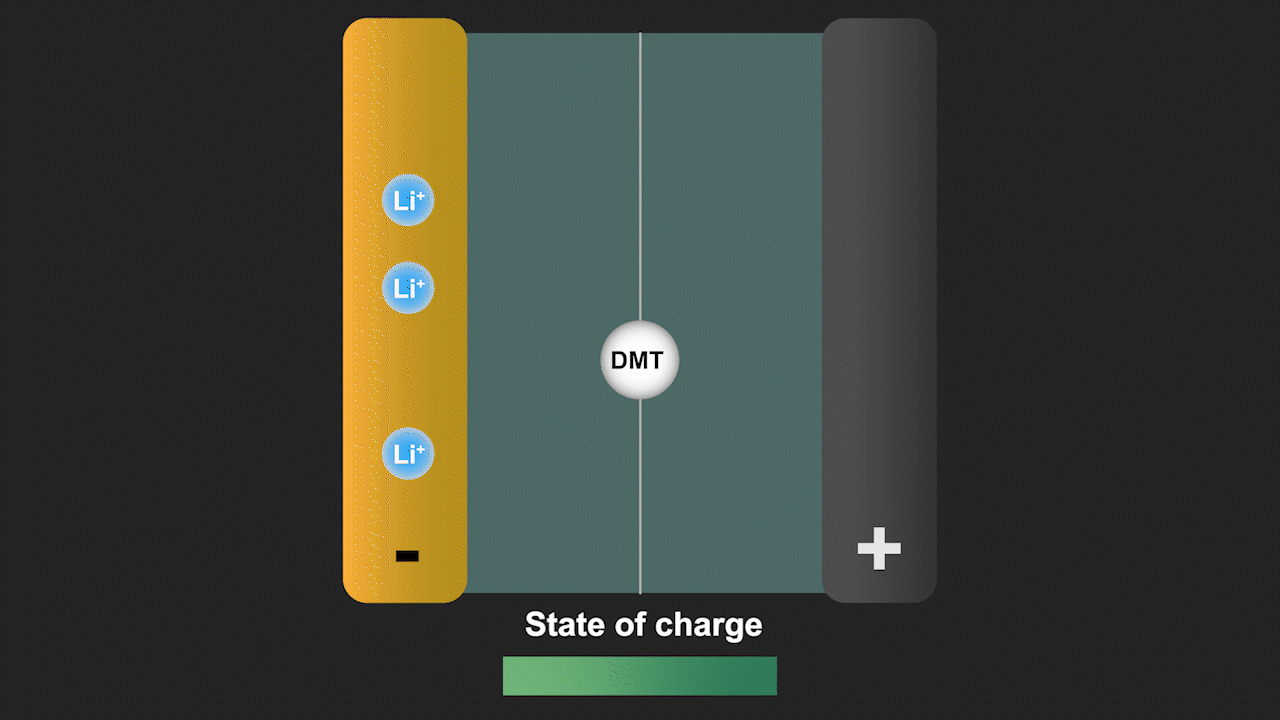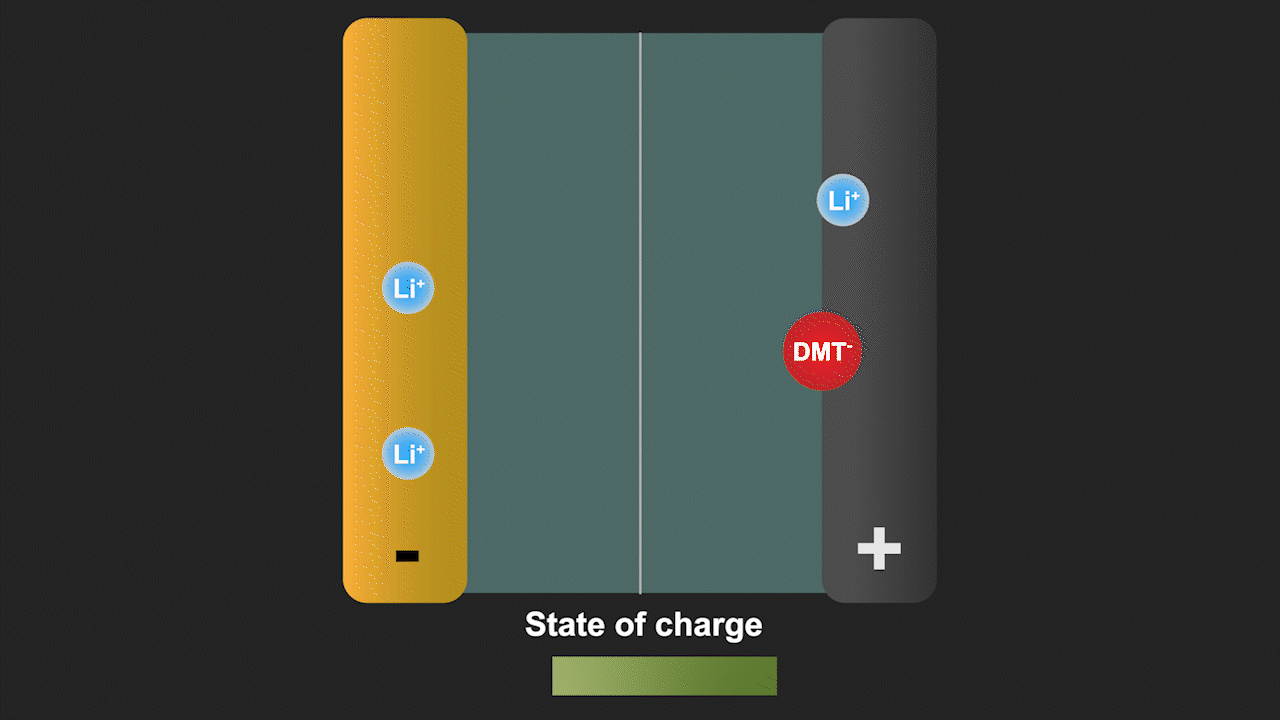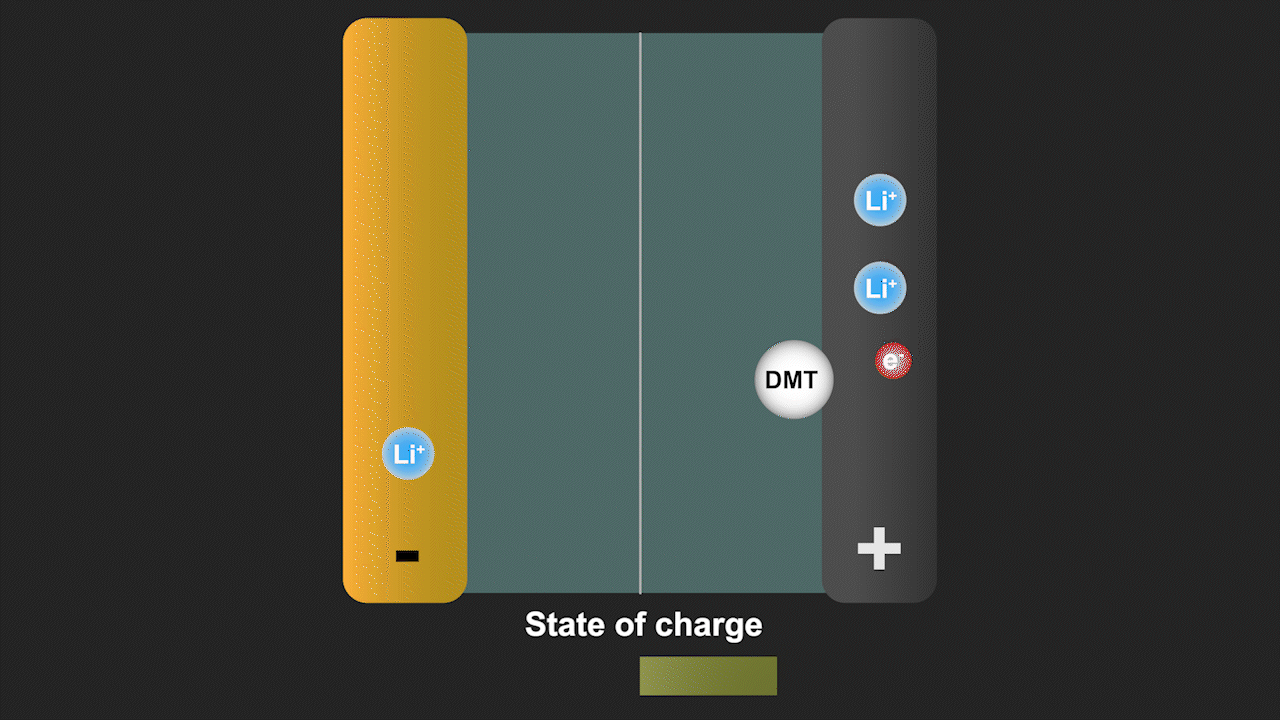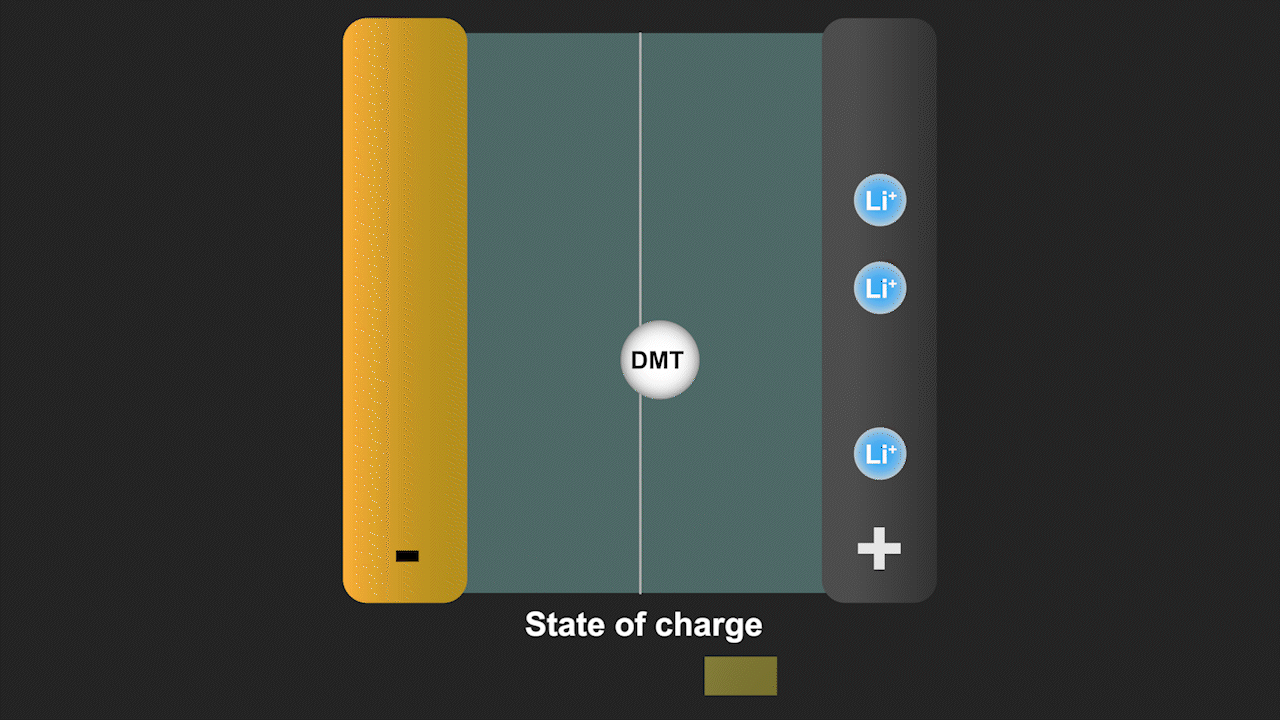
Figure 3. Redox shuttle mechanism of the molecule dimethyl terephthalate (DMT) identified by our group leading to self-discharge of battery cells without effective electrolyte additives.
[1] E. Lyle, R. Väli, A. Dutta, and M. Metzger, Melt Synthesis of Lithium Manganese Iron Phosphate: Part I. Composition, Physical Properties, Structural Analysis, and Charge/Discharge Cycling. J. Electrochem. Soc. 169, 060526 (2022). https://doi.org/10.1149/1945-7111/ac76e4
[2] C. Aiken, E. R. Logan, A. Eldesoky, H. Habecker, J. M. Oxner, J. E. Harlow, M. Metzger, and J. R. Dahn, Li[Ni0.5Mn0.3Co0.2]O2 as a Superior Alternative to LiFePO4 for Long-Lived Low Voltage Li-Ion Cells. J. Electrochem. Soc. 169, 050512 (2022). https://doi.org/10.1149/1945-7111/ac67b5
[3] T. Boulanger, A. Eldesoky, S. Buechele, T. Taskovic, S. Azam, C. Aiken, E. R. Logan, and M. Metzger, Investigation of Redox Shuttle Generation in LFP/Graphite and NMC811/Graphite Cells. J. Electrochem. Soc. 169, 040518 (2022). https://doi.org/10.1149/1945-7111/ac62c6
Research Highlight
Changes to lithium-ion battery electrolytes can induce self-discharge via a redox shuttle reaction:


2. Sodium-ion Batteries
Next generation battery cells do not have to rely on lithium. Sodium-ion batteries represent an attractive alternative to lithium-ion batteries as sodium is high in abundance and low in cost. Since the energy density of sodium-ion batteries is low, the electrification of the transport sector still requires sustainable lithium-ion batteries. However, sodium-ion batteries are suitable for stationary storage of renewable energy, which may become even more important than sustainable mobility.
Why we develop sodium-ion batteries
There is no doubt that lithium is the best element for rechargeable batteries in terms of its electrochemical properties. However, the earth's lithium reserves are limited, unevenly distributed, and as we "electrify everything" the lithium production may not be able to keep up with the increase in demand (see Figure 4). Lithium mines that started operations between 2010 and 2019 took an average of 16.5 years to develop, putting in question how rapidly new lithium mining capacity can come online to meet the expected rise in demand. The logical conclusion it to develop an alternative battery concept that uses sodium - an element with similar properties as lithium, but far higher abundance (see Table I).

Figure 4. This lithium supply and demand balance prediction shows that even in a very high lithium production scenario, we could run into supply deficits soon. [2]
Table I. Comparison between the characteristics of lithium and sodium. [2]
Metal | Standard electrode potential (V vs. SHE) | Theoretical capacity (mAh/g) | atomic weight (u) | Ionic radius (nm) | Cost ($ per t) | Percentage in the earth's crust |
|---|---|---|---|---|---|---|
sodium | -2.71 | 1166 | 22.99 | 0.98 | 150 | 2.83% |
lithium | -3.05 | 3862 | 6.94 | 0.69 | 5000 | 0.01% |
Challenges for sodium-ion batteries
Unfortunately, we cannot expect to just put sodium in our batteries instead of lithium and get the same results. First, sodium ions are bigger and heavier than lithium ions, which leads to slow reaction kinetics. Second, sodium has a higher standard electrode potential than lithium, which leads to lower operating voltage and lower energy density. Thus, a larger battery cell is needed to store the same amount of energy (see Figure 5). Moreover, lithium-ion batteries profit from a very important passivation layer on the negative electrode (the so-called solid electrolyte interphase or SEI). In sodium-ion batteries the SEI is somewhat less stable and a general understanding of this important component is currently lacking.

Figure 5. Comparing the thickness of lithium-ion and sodium-ion cells. These cells have approximately the same energy content, but the Na-ion cells are much thicker, illustrating their low energy density.
On the bright side, sodium-ion cells can be made from abundant and low-cost manganese and iron for the positive electrode and hard carbon derived from various eco-friendly precursors, e.g., biomass, for the negative electrode. In our lab we synthesize these materials, process them into electrodes, pair them with our own electrolyte formulations and try to find out which materials combinations are best suited for long-lived sodium-ion cells.
[1] P. Greim, A.A. Solomon, and C. Breyer, Assessment of lithium criticality in the global energy transition and addressing policy gaps in transportation. Nature Communications 11, 4570 (2020). https://doi.org/10.1038/s41467-020-18402-y
[2] F. Li, Z. Wei, A. Manthiram, Y. Feng, J. Ma, and L. Mai, Sodium-based batteries: from critical materials to battery systems. J. Mater. Chem. A 7, 9406–9431 (2019). https://doi.org/10.1039/C8TA11999F
Research Highlight
Sodium plating is an issue in Na-ion cells, just like lithium plating in Li-ion Cells; We studied the condictions under which sodium plating sets in and present electrolytes that prevent sodium plating:
H, Hijazi,= Z. Ye,= L. Zhang, J. Deshmukh, M. B. Johnson, J. R. Dahn, and M. Metzger, Impact of Sodium Metal Plating on Cycling Performance of Layered Oxide/Hard Carbon Sodium-ion Pouch Cells with Different Voltage Cut-offs, accepted (2023).

3. New Methods
The cell chemistry in advanced battery cells is highly complex and important degradation reactions are often poorly understood. In this section, we will tell you about our research equipment and novel characterization methods we develop in our lab to understand battery degradation and enable new materials discoveries.

On-line Electrochemical Mass Spectrometry (OEMS)
Interfacial reactions in lithium-ion batteries often involve gaseous reaction products. OEMS is an advanced battery gas analysis technique that allows us to identify and quantify the gaseous species that are created during the charge and discharge process of a battery (see Figure 6). The OEMS system consists of a ultra-high vacuum chamber connected to an array of battery cells on one side and a high-precision mass spectrometer on the other side. Measurements take place continuously while the battery cells are cycling ("on-line" refers to "while the cell is operating"). Only when we understand which side reaction causes a battery cell to deteriorate, we can do something about it. This is exactly where the OEMS system comes is as it helps us to identify unwanted gaseous reaction products and establish a mechanism for how they are created.

Figure 6. This figure shows gas data generated by OEMS during the first cycles of a battery cell (formation cycles). The OEMS system tracked the creation of the gases hydrogen (H2), ethylene (C2H4), carbon monoxide (CO), and carbon dioxide (CO2) during cell operation. Additives are very effective at reducing gas generation. [1]
Prof. Metzger studied fundamental materials degradation mechanisms in lithium-ion batteries via OEMS in his dissertation. At Dalhousie, we will take this method to the next level and apply it to real-world prototypes of lithium and sodium-ion cells. Furthermore, long-term studies of gas generation and consumption in batteries will be important, as will be gas analysis in long-lived cells with mature interfaces.
[1] M. Metzger, B. Strehle, S. Solchenbach, and H. A. Gasteiger, Origin of H2 Evolution in LIBs: H2O Reduction vs. Electrolyte Oxidation. J. Electrochem. Soc. 163, A798–A809 (2016). https://doi.org/10.1149/2.1151605jes
Research Highlight
On-line electrochemical mass spectrometry can be used to differentiate between gases generated at the anode, the cathode or through crosstalk between the electrodes of lithium-ion batteries:

4. Desalination Batteries
The materials and principles used for sodium-ion batteries (see paragraph above) can be used to design novel devices for energy-efficient water desalination. In this project, we use the intercalation of alkali metals into crystalline host structures – a concept well known from the battery field – to remove salt from sea water.
How batteries can solve a water crisis
Water scarcity is a growing problem and already today about four billion people worldwide suffer from limited availability of freshwater. Like energy security, water security is paramount in our daily lives and essential for manufacturing and agriculture industries that heavily dependent on it. Despite over two-thirds of our planet being covered with water, only one percent exists in the form of freshwater and the majority is seawater, which requires energy input to reduce its salt content for human use. State-of-the-art desalination technologies like reverse osmosis or thermal desalination aim to mitigate this by converting seawater into freshwater, but suffer from energy-water inefficiencies, membrane fouling, greenhouse gas emissions and other shortcomings.
Desalination batteries are a promising technology that uses battery-inspired materials to overcome the afore-mentioned drawbacks. Like in any typical lithium or sodium-ion battery, ions get inserted into or removed from crystalline host materials during charge and discharge as a function of applied current or voltage. This time, however, the goal is to desalinate seawater – hence the name “Desalination Battery”. Our group primarily investigates so-called Prussian Blue Analogues for desalination batteries and we go all the way from materials synthesis to testing in flow cells with the goal to bring this technology one step closer to realization.
Research Highlight
Performance and Lifetime of Battery Desalination Cells Based on Nickel Hexacyanoferrate: